Relieving Devising and Creating Life-Saving Medical Equipment Innovations
The healthcare technology sector is shifting more towards the incorporation of AI-powered tools and enabling the utilization of implantable sensors for diagnostic purposes. The innovation in the medical devices market is indeed astonishing. At Prism Computer Solutions, we focus on devising and engineering medical devices at PCS, which comply with industry standards and cover all regulatory obligations.
Step-By-Step Compliance: Our Strategy
Idea conceptualization has innovated at a staggering pace, especially when looking to design a medical device. However there are rules that one must follow that govern ideas. In short, the process of coming up with a medical device is far more complicated. Here is how we go about it:
✔️ Concept & Feasibility Study: Technical requirements can be evaluated alongside market opportunities.
✔️ Design & Prototyping: Our sodail team aims at developing new efficient and predominantly safe functional prototypes.
✔️ RF Testing & Compliance: Federal Communication Commission, FDA, European Economic Area approvals are first obtained before devices earning international market MS compliance.
✔️ Final Certification & Manufacturing: Product labeling, required documentation, and guides are sold alongside product launches.
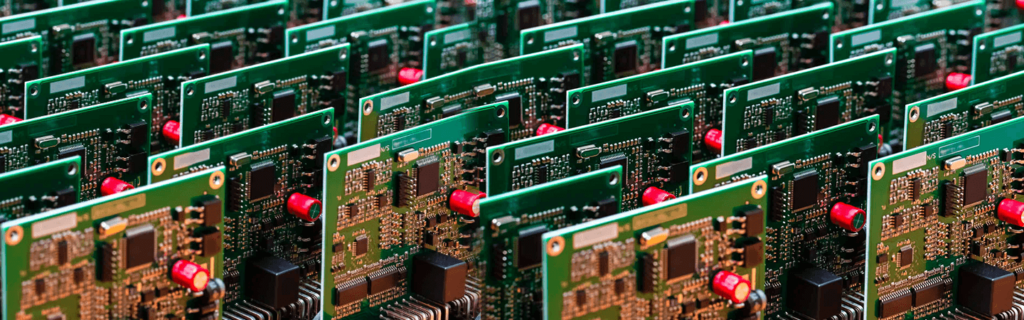
Our Work’s Value Portrait
Precision, reliability, and compliance—ensuring your PCB boards meet the highest industry standards through rigorous testing.
Our experience is deeply implantable, externally worn and additional internal medical devices meant to enhance the patient experience while simultaneously bringing efficiency to the healthcare sector. From wireless ECG gadgets to remote monitoring systems, we seek to aid corporations with novel solutions.




Comments are closed